Covid19: J&J Vaccine Rollout to Resume, Over 1k New Cases Reported
- The rollout of the Johnson & Johnson Covid-19 vaccine will resume from Wednesday, 28 April in the country
- The South African Health Products Regulatory Authority stated that it did not find any blood clots or blood disorders in South Africa
- Phase 1 of the vaccine will be concluded on 16 May but those taking part will need to give consent once again
PAY ATTENTION: Click “See First” under the “Following” tab to see Briefly.co.za News on your News Feed!
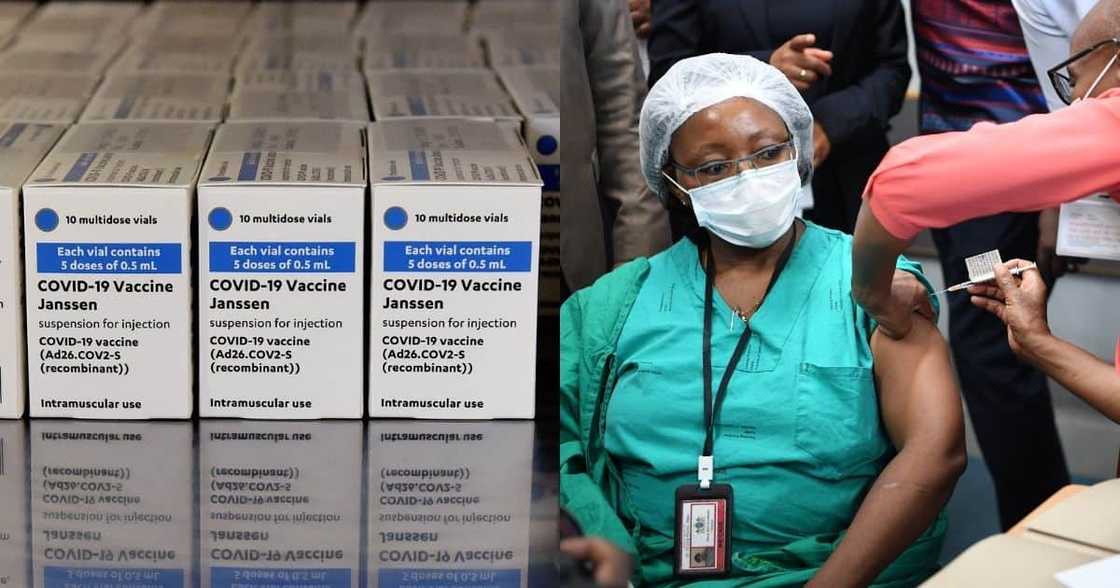
The Johnson & Johnson Covid-19 vaccine rollout in South Africa will be resuming from Wednesday, 28 April. The vaccine was put on hold after a few recipients in the US were found to have developed rare blood clots after getting vaccinated.
The South African Health Products Regulatory Authority (SAHPRA) reportedly did not find any evidence of the blood clots forming in South Africans who had received the J&J vaccine. Reports say that health authorities believe there is a one-in-a-million chance of developing a blood clot.
The country is still in Phase 1 of the Sisonke Vaccination Programme, which involves healthcare workers. Phase 1 is expected to conclude on 16 May.
Source: Getty Images
According to eNCA, vaccination sites in the country will be expanded to 95 sites. The report continued by saying that those partaking in the programme will be once again required to give consent.
Health authorities have, however, recommended that lactating and pregnant women be excluded from the vaccine programme.
New cases of Covid-19
1 101 new Covid-19 cases have been reported while 23 new daily deaths have unfortunately been recorded.
Summary
The cumulative number of confirmed Covid-19 cases in South Africa is 1 575 471 while the total number of deaths is 54 148.
Previously, Briefly News reported that the government had lifted the suspension of the Johnson & Johnson Covid-19 vaccine. Acting Minister in Presidency Khumbudzo Ntshaveni confirmed that South African scientists reviewed the vaccine and found that it was not harmful despite claims that it was causing blood clots after six patients in the US are said to have developed a rare type of blood clot after being administered the vaccine.
She has announced that the roll-out of the vaccine will continue at a later date when the department is ready. She also added that the pause in the roll-out of the vaccine was part of a necessary precaution to ensure the safe administration of Covid-19 vaccines to South Africans.
The news follows the South African Health Products Regulatory Authority proposing that the government terminate the pause on the administering of the Johnson & Johnson's Covid-19 vaccines, provided that certain conditions are met.
Enjoyed reading our story? Download BRIEFLY's news app on Google Play now and stay up-to-date with major South African news!
Source: Briefly News



